Hydrogen Peroxide Overview


Hydrogen peroxide chemical formula is H2O2 and it is the cleanest oxidizing agent on decomposition giving oxygen and hydrogen. It is oxidizing and reducing agent but its reducing power is low so mainly used as oxidizing and bleaching agent in industries. It is very unstable when used in high concentrations and deteriorates over time so some external agent has to be used to stabilize hydrogen peroxide which is mainly an organic compound. Contact to this chemical severely effect mucus membrane, skin, eyes and other parts of the body.

Manufacturing Process
Production of Hydrogen Peroxide:
Electrolysis of Ammonium bisulphate and sulphuric acid:
Here in this process a large amount of electrolysis of ammonium bisulphate in sulphuric acid is done to produce sulphuric acid solution of ammonium persulphate which is then treated under distillation with subsequent cooling which then reaction with water forms hydrogen peroxide. In this process the residual is again send to for electrolysis and cycle is repeated. The overall reaction for the process is given below.
Autoxidation Process:
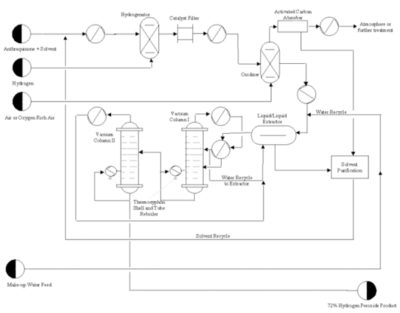
These days, it is manufactured by the anthraquinone process also known as AO i.e. Autoxidation process. Hydrogen peroxide is produced using hydrogen and oxygen from air and the reaction is done over anthraquinone which acts as a reaction carrier here. Steps of the reaction are as follows-
- Anthraquinone with steam containing Hydrogen based carrier solvent (work solution) is fed into the hydrogenator reactor where hydrogenation reaction occurs over Ni and Pd catalyst gives Anthraquinol.
- Before sending the product from 1st reactor to 2nd reactor referred as oxidizer are filtered and cooled. The oxygen enriched air or oxygen with Anthraquinol is fed to second reactor where oxidation reaction occurs gives anthraquinone and hydrogen peroxide.
- Hydrogen peroxide is separated from work solution containing 40% hydrogen peroxide after cooling using liquid-liquid extraction where water is used as a medium.
- Hydrogen peroxide is sent vacuum column and Anthraquinone is then sent to purifier it is reactivated before send to recycling unit.
- Product from vacuum is preheated using steam and sends to the extractor to remove water-hydrogen peroxide mixture there may be multiple extractors depending upon the desired purity required. Distillation is used to get purity up to 70%. Inhibitors are added to hydrogen peroxide to avoid its reaction with metals.

The final reaction is as follows:

Hydrogen Peroxide Applications
Pulp and Paper industry:
Hydrogen peroxide is oxidizing as well as reducing agent which broaden its field of application. It is mainly used as a bleaching agent in the pulp and paper industry during chemical and mechanical pulping process. In chemical bleaching, H2O2 provides alkaline medium to the system due to environmental effects it can be prove as a great replacement. Hydrogen peroxide can be used to produce TCF and ECF pulps and it also increase the strength of the paper and pulp.
Textile industry:
Hydrogen peroxide is used as a bleaching agent in textile industry and they are more environmental friendly than the chlorine-based bleaches. H2O2 is also used in dyes. Sulphur dyes work with an oxidation-reduction dyeing method and hydrogen peroxide is used as an oxidizing agent. Once the dyes are adsorbed onto the cellulosic, oxidation will settle the dyes permanently and changing it into a water-insoluble form.
Chemical Industry:
Hydrogen Peroxide is used for manufacturing chemicals such as Sodium Percarbonate and Sodium Perborate, which are in turn used for the bleaching action of laundry detergents. It is also used in the production of certain organic peroxides like dibenzoyl peroxide. These organic peroxides are used to treat acnes, are used in polymerisation reactions and as a flour bleaching agent. They can even be used to produce peroxy acids such as peracetic acid and meta chloroperoxybenzoic acid.
Waste water treatment:
Hydrogen peroxide is very powerful oxidizing agent in water treatment it leaves no residues like other chemicals. It is used in waste water treatment processes to remove organic impurities and is able to remove organic contaminants such as aromatic or halogenated compounds, which are otherwise very difficult to remove. It can also oxidise sulphur based compounds that are present in the waste, thus reducing the foul odour.
Cosmetic applications:
Hydrogen peroxide is useful in hair care products, hair dyes, conditioner, shampoos, hair bleaches and tooth whitening products. In cosmetic it can be used as a oxidizing and antimicrobial agent which inhibits the growth of microorganism also used as an antiseptic agent. During its use in hair dye it forms dyestuffs in oxidative hair dyeing. Diluted hydrogen peroxide is mixed with ammonium hydroxide and used in the bleaching of hair. It is also used in the whitening of teeth and is often used in homemade toothpastes. It is also used to treat acne.
Medical applications:
Hydrogen peroxide is used as a disinfectant and is eco-friendly, when compared to other chlorine based bleaches. It is also recognised by the FDA as a safe antimicrobial agent that can be used to disinfect various surfaces. Practitioners of alternative medicine use hydrogen peroxide for the treatment of various conditions including emphysema, influenza, AIDS and cancer. However, large doses of hydrogen peroxide can result in blistering and irritation, diarrhoea, abdominal pain and vomiting. Hence, the treatment is not approved by the US FDA.
Other important applications:
Hydrogen Peroxide is used as a propellant in rockets. It can either be used as a monopropellant or as the oxidiser component of a bipropellant rocket. It is also used to make organic peroxide based explosives, for example, acetone peroxide. However, these explosives degrade very soon, hence cannot be used for commercial applications. It is used in horticulture, in extremely diluted forms, for watering plants. It is believed to enhance the development of the plant’s roots and prevents the rotting of roots by supplying enough oxygen for the growth of cells. Another application of hydrogen peroxide is in providing aeration for fish. The mechanism behind the use of hydrogen peroxide is its decomposition when exposed to catalysts such as manganese dioxide, thus releasing oxygen.
Hydrogen Peroxide Specifications
| PRODUCT DETAILS | ||
| CAS No. | 7722-84-1 | |
| Molecular Formula | H2O2 | |
| Molecular Weight | 34.0147g/mol | |
| IUPAC Name | Hydrogen Peroxide | |
| PHYSICAL AND CHEMICAL PROPERTIES | |
| Physical State | WhiteLiquid |
| Odor | Sharp |
| density | 1.11 g/cm3 20oC, 30% |
| Viscosity | 1.245 cP (20 °C) |
| Melting Point | −0.43 °C |
| Boiling Point | 150.2 °C |
| PH | 1.5 |
| Refractive index | 1.4061 |
| SALES SPECIFICATION | ||
| Purity H2o2,% by wt | 35% | 50% |
| Density | 1.13 gm/cm3 at 20oC | 1.20 gm/cm3 at 20oC |
| Stability, % by wt | 99.6% | Min. 98% |
| water | 65% | 50% |
| Free acid, % by wt | Max. 0.03 | |
| pH | Max. 2.0 at 20°C | 2-3 at 20OC |
| Density | 1190 kg/m3 at 25°C | 2-3 at 20OC |
